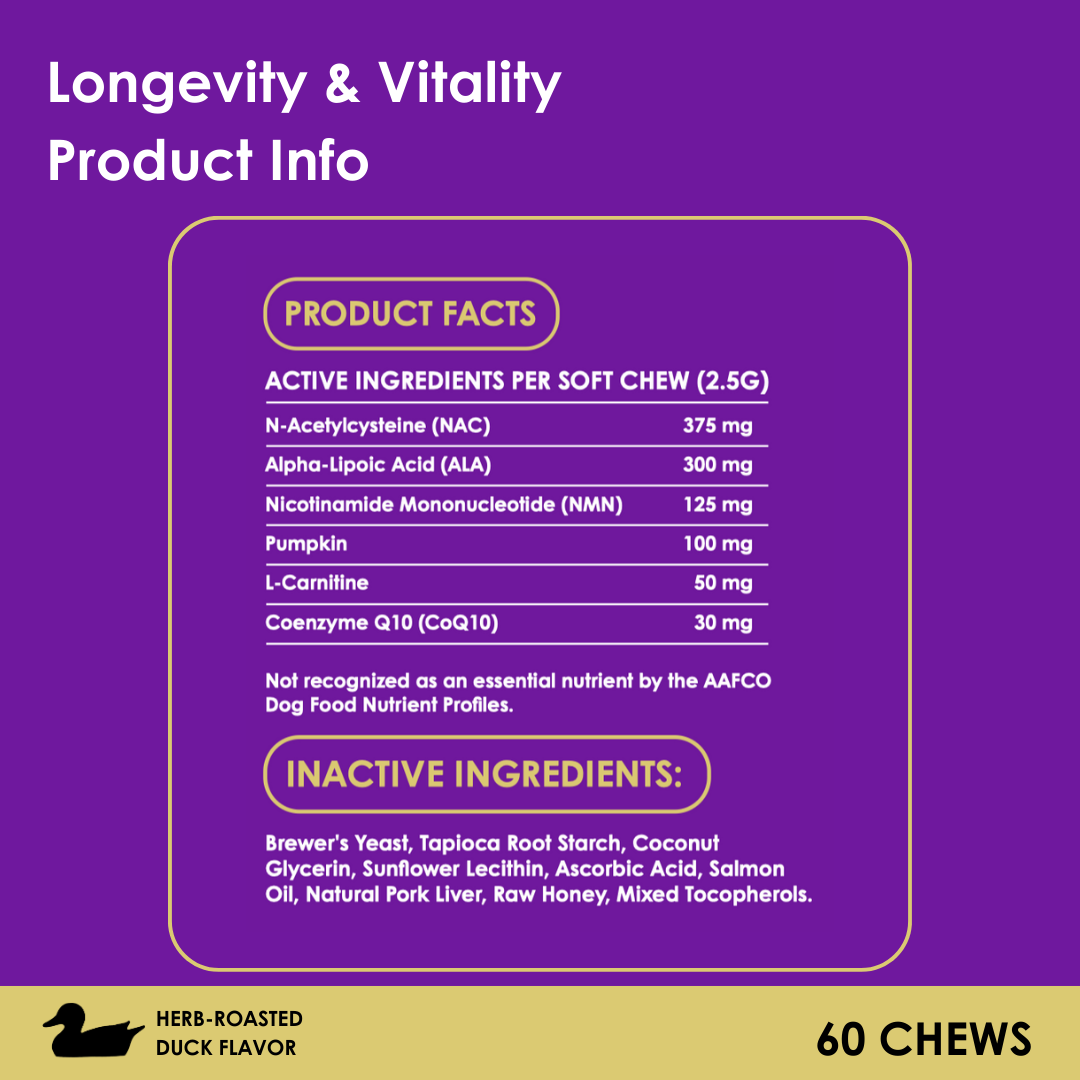
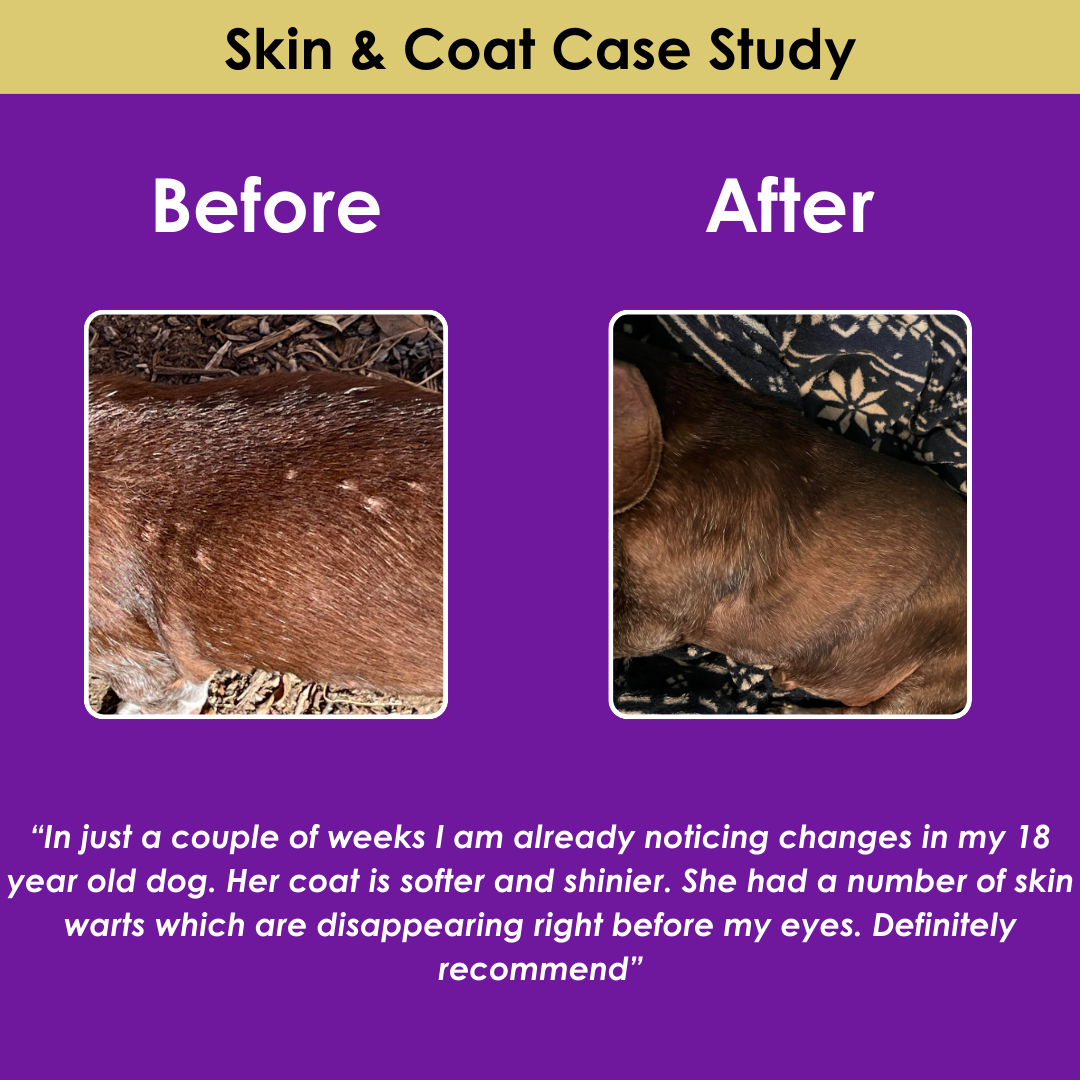
FDA Approves New Oral Parasite Medication for Dogs
In October 2024, the FDA approved a new oral parasiticide designed to protect dogs from six types of common parasites. The chewable tablet, which contains a combination of lotilaner, moxidectin, praziquantel, and pyrantel, provides monthly protection against fleas, ticks, heartworms, roundworms, hookworms, and three species of tapeworms. This approval marks the introduction of the first product to combine four active ingredients to offer such broad-spectrum coverage.
Key Details of the FDA-Approved Medication
The new chewable tablet, manufactured by Elanco Animal Health under the brand name Credelio Quattro, is suitable for dogs as young as 8 weeks and weighing as little as 3.3 pounds. It is designed to be administered once a month, providing consistent, long-lasting protection against both external parasites like fleas and ticks, as well as internal parasites that pose a zoonotic risk (those capable of spreading to humans).
Notable features of the product include:
- The ability to kill ticks within 4 hours and more than 99% of fleas within 8 hours of administration, maintaining efficacy throughout the month.
- Proven 100% protection against heartworm disease in laboratory studies.
- Coverage against zoonotic parasites, such as echinococcus tapeworms, which can potentially infect both pets and people.
Why Parasite Protection Matters for Dog Longevity
Parasites pose significant health risks to dogs, ranging from discomfort and skin irritation to life-threatening conditions like heartworm disease or serious internal infections caused by roundworms and hookworms. Additionally, some parasites can spread from pets to humans, making year-round protection a public health consideration.
Preventing parasite infestations is essential not only for maintaining a dog's immediate health but also for supporting their long-term longevity. Chronic infestations, if untreated, can lead to significant health complications that reduce a dog's quality of life and lifespan. By using effective parasite preventives like this FDA-approved chewable tablet, dog owners can help ensure their pets live healthier, longer lives.
Though the product is expected to launch in early 2025, pet owners are encouraged to consult their veterinarians to determine the most suitable parasite prevention plan for their dogs, especially in areas where parasite pressure is high due to environmental factors like rising temperatures.