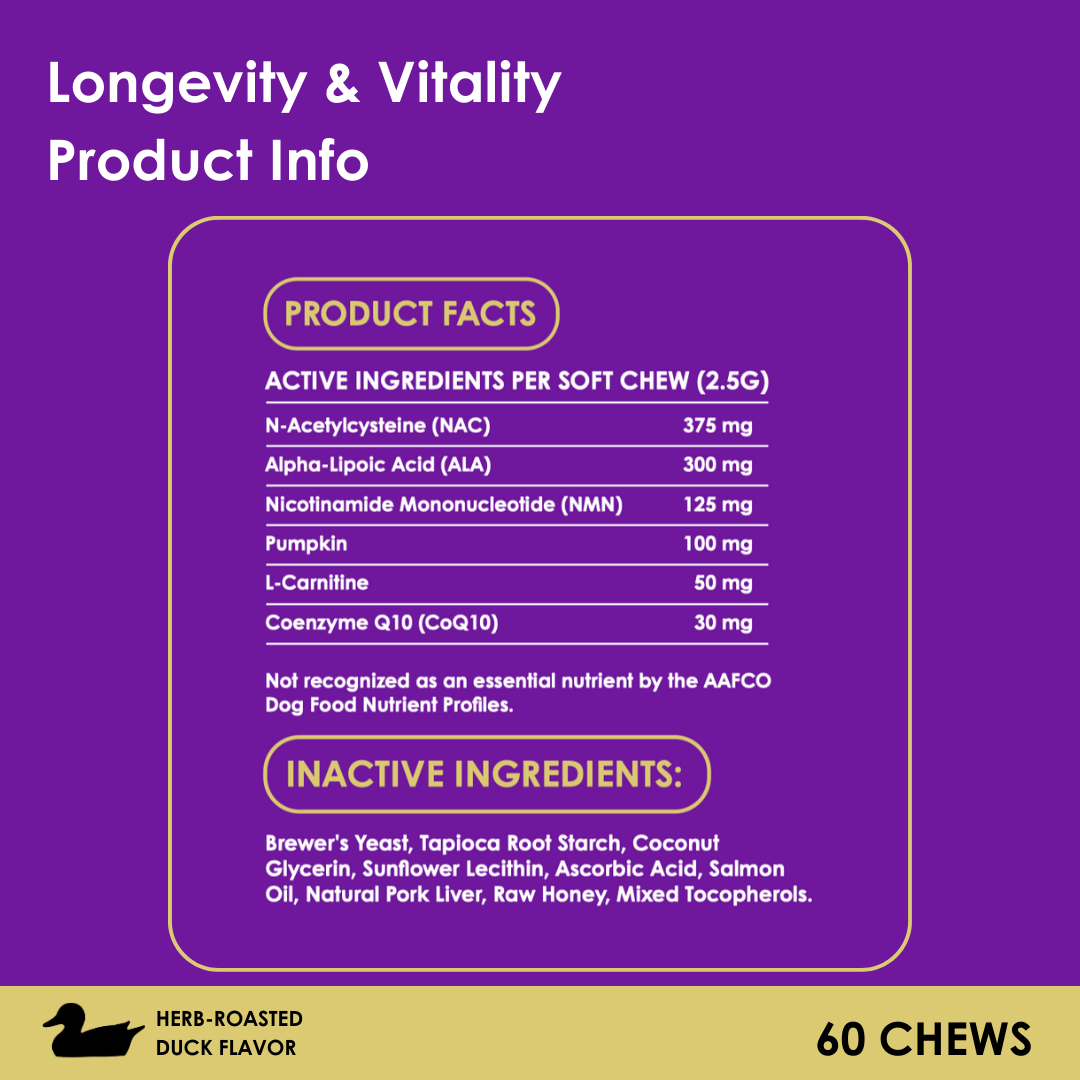
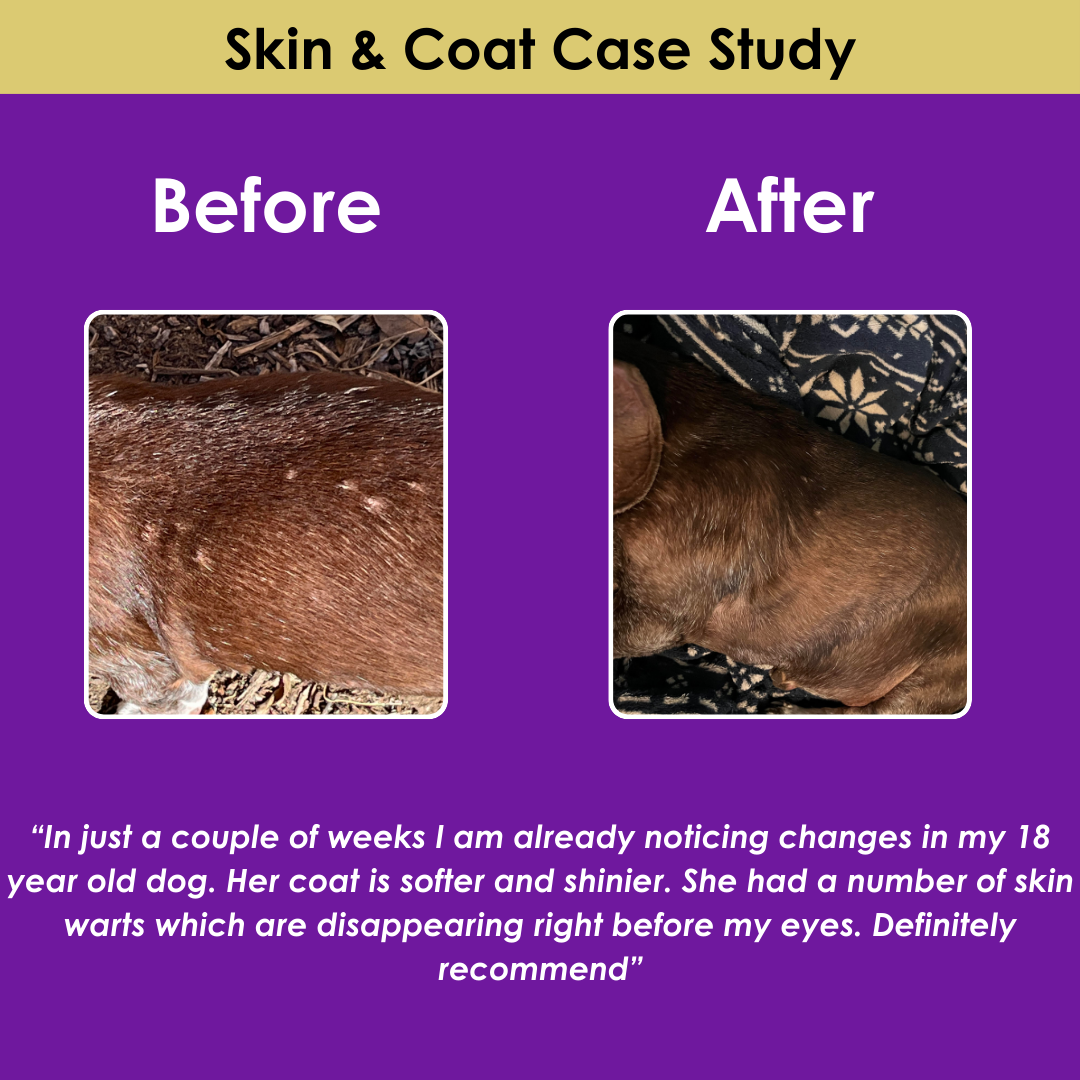
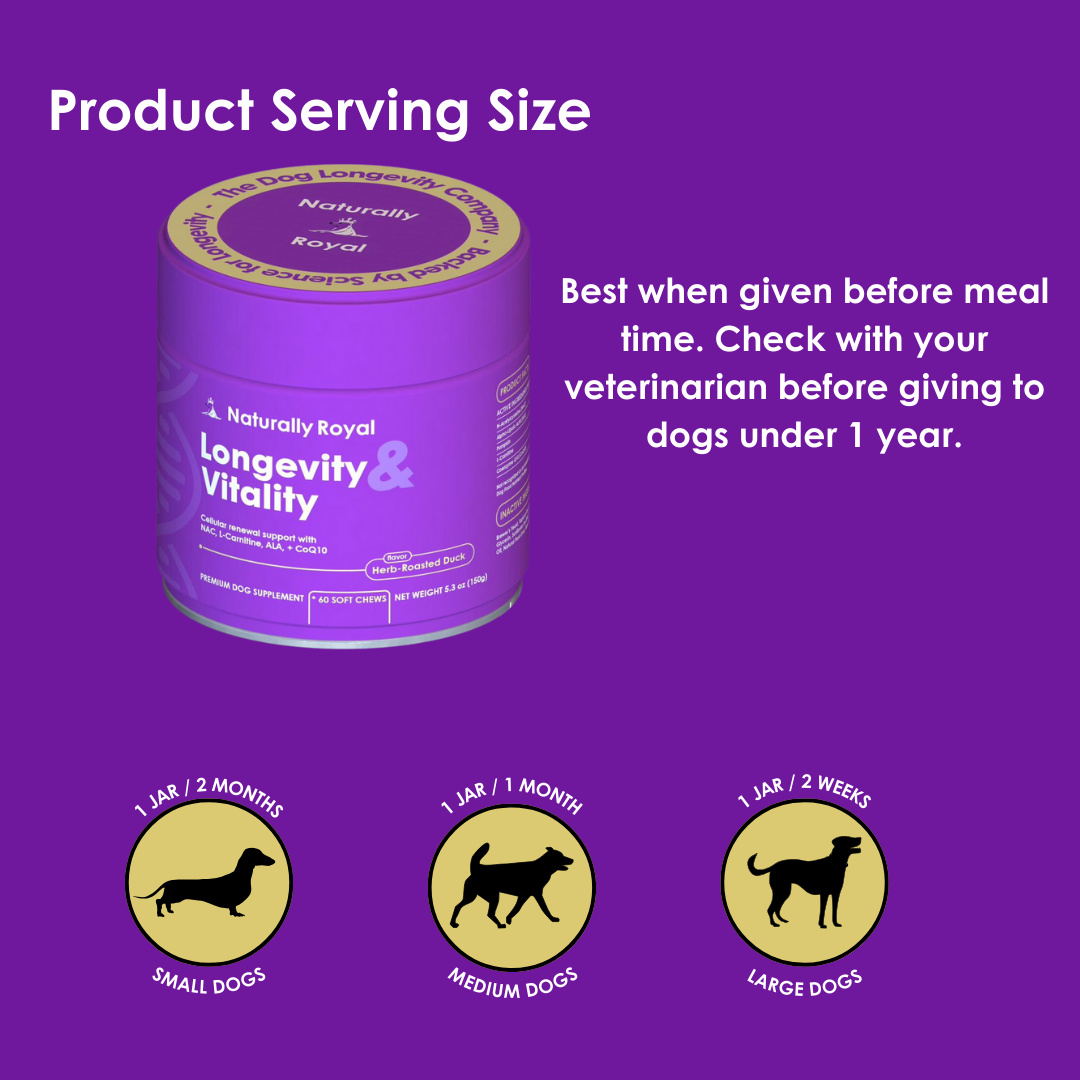
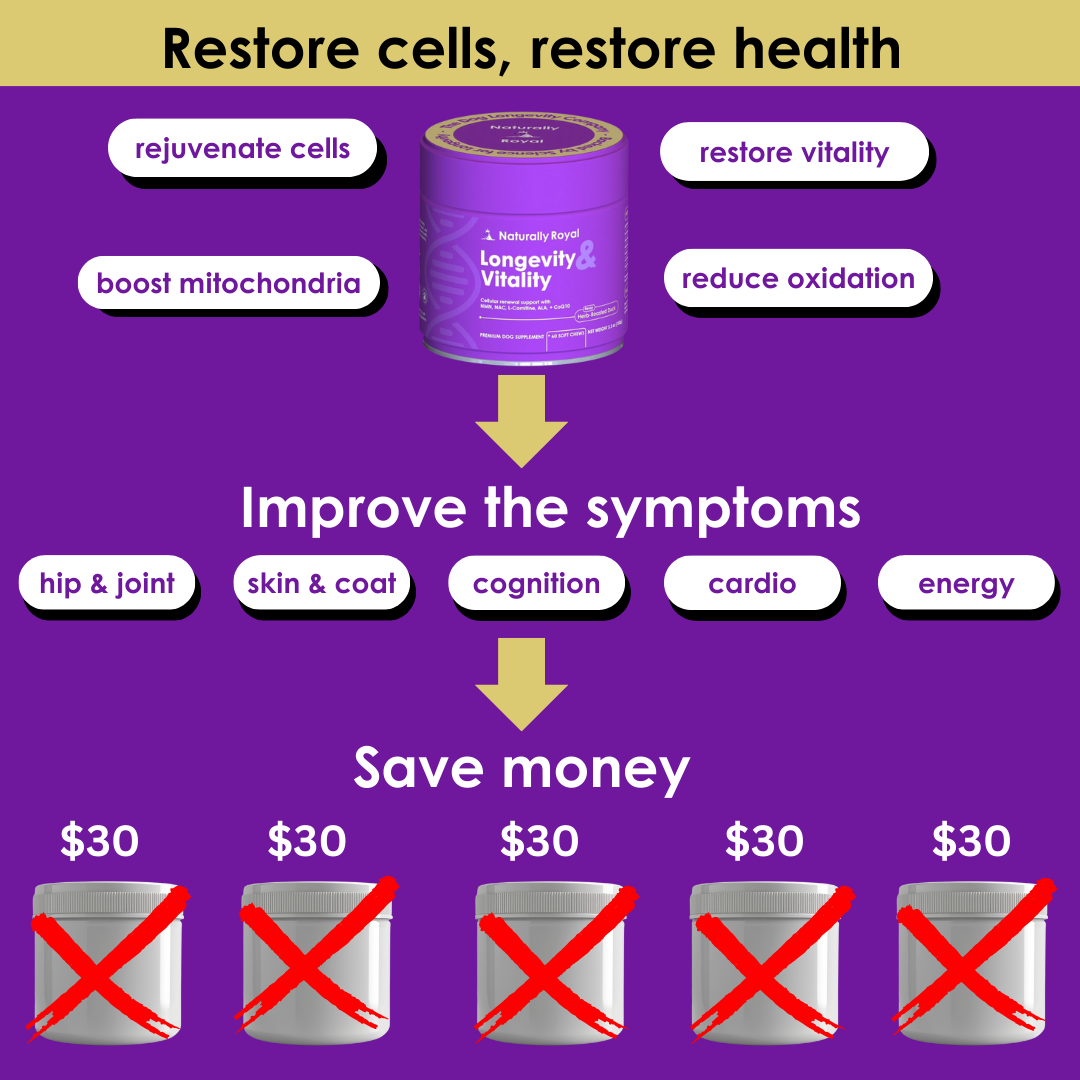
FDA Approves First Generic Heart Failure Medication
The FDA's recent approval of pimobendan chewable tablets marks a significant milestone in veterinary medicine. This approval introduces the first generic version of the heart failure medication, previously known under the brand name Vetmedin by Boehringer Ingelheim. Let’s go over what the drug is, what it treats, and the implications for longevity:
About Pimobendan:
Pimobendan is classified as an inodilator, which means it has dual action: it strengthens the heart’s contractions and dilates the blood vessels. This combination helps the heart pump more efficiently, alleviating the symptoms of heart disease and extending the quality of life for dogs with specific heart conditions.
Conditions Treated:
The FDA has approved pimobendan for managing mild, moderate, or severe congestive heart failure in dogs, specifically those caused by myxomatous mitral valve disease (MMVD) or dilated cardiomyopathy (DCM). MMVD is more common in small breed dogs like Cavalier King Charles Spaniels, Yorkshire Terriers, and Dachshunds, while DCM is often seen in larger breeds and has a genetic component.
Implications for Longevity:
-
Extended Quality of Life:
- By improving heart function and reducing symptoms, pimobendan can significantly extend the quality of life for dogs with heart disease. This means more healthy years with reduced symptoms related to heart failure.
-
Preventative Care:
- For breeds predisposed to MMVD or DCM, early and proactive use of pimobendan might slow the progression of heart disease, potentially preventing the onset of congestive heart failure.
-
Cost-Effective Treatment:
- The introduction of a generic version makes this critical treatment more accessible and affordable for dog owners, ensuring more pets can benefit from this life-extending medication.
Administration and Availability:
Pimobendan chewable tablets are prescribed by a veterinarian and should be used in conjunction with other treatments for congestive heart failure as deemed appropriate by a veterinary professional. The drug is administered orally, twice a day in doses calculated based on the dog's weight. It is available in various strengths to accommodate different sizes of dogs and specific dosing needs.
The FDA approval of generic pimobendan is a welcome development in veterinary medicine, offering a more accessible and affordable option for managing dog heart diseases. By effectively treating heart conditions, pimobendan not only improves the quality of life but also extends the lives of affected dogs, providing them with more healthy years alongside their families.